- China
- English
- Françai
- Español
- Deutsch
- Română
- العربية
- 한국어
- 日本語
- Italiano
- Português
- Gaeilge
- Dansk
- Čeština
- Русский
- Afrikaans
- Euskara
- Català
- Esperanto
- हिन्दी
- Ελληνικά
- Bahasa Melayu
- Polski
- Српски
- Kiswahili
- ภาษาไทย
- Tiếng Việt
- Türkçe
- Svenska
- Cymraeg
- Slovenčina
- Latviešu
- Malti
- Magyar
- Galego
- ગુજરાતી
- Eesti Keel
- বাংলা
- Shqip
- беларуская мова
- Nederlands
- Tagalog
- ქართული
- Íslenska
- Kreyòl Ayisyen
- Lietuvių
- Norsk
- slovenščina
- தமிழ்
- Українська
- ײִדיש
- اردو
- తెలుగు
- فارسی
- македонски
- ಕನ್ನಡ
- Bahasa Indonesia
- עברית
- Suomi
- Hrvatski
- Български
- Azerbaijani

Industry Application
Product Type
How To Stop Lithium-Ion Batteries Explosion Due To Thermal Runaway?

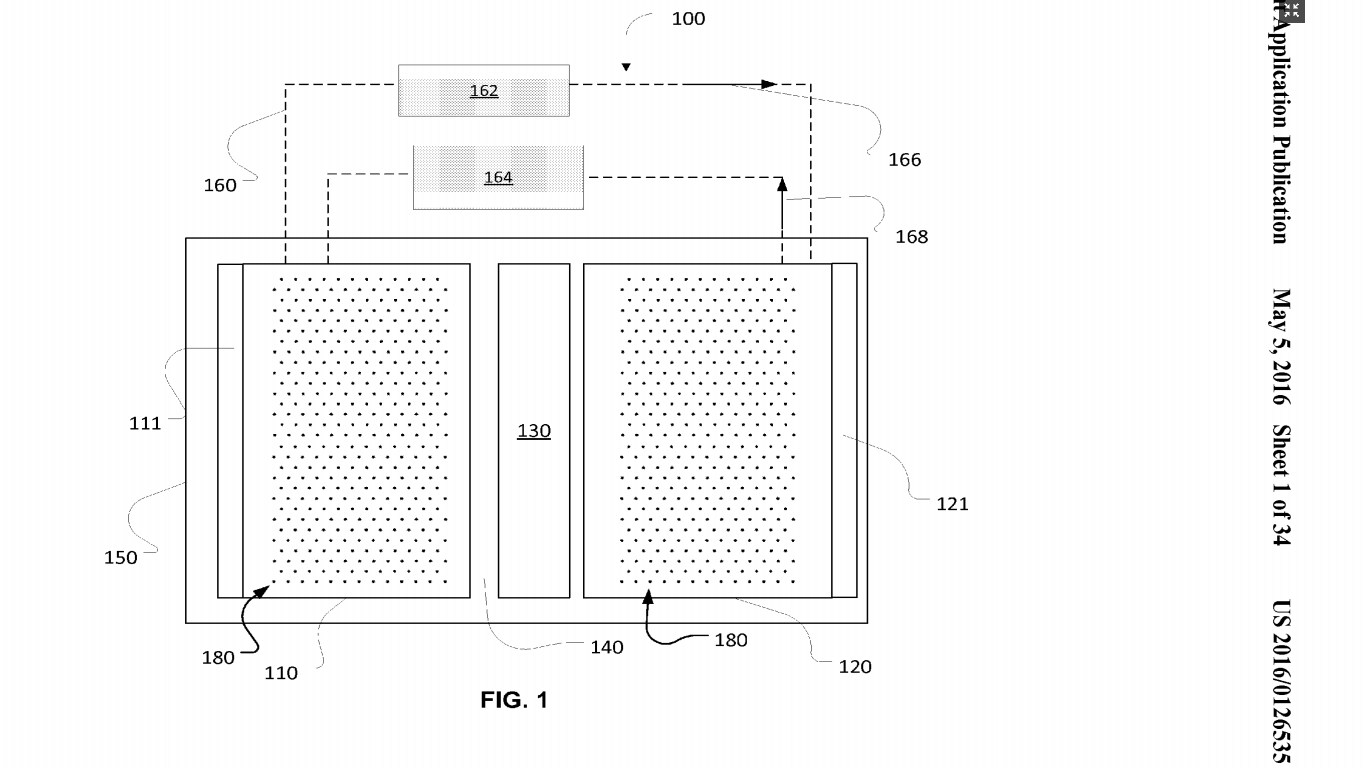
Thermal runaway is a long standing problem that has bugged large corporations like Tesla, Samsung, and Boeing and small alike. Boeing’s Dreamliner 787, which Boeing advertised as 20% fuel efficient, was grounded in 2013. In the same year, Tesla’s Model S came under a federal safety investigation after it caught fire at least 3 times. Last year Samsung recalled 2.5 million Galaxy Note 7 smartphone. For all the three companies, which are top players of their domain, the problem was same – the Lithium-Ion batteries installed in heart of their product as a power source. The lithium-ion batteries installed in Tesla Model S, Dreamliner 787 and Galaxy Note 7 were exploding continuously. Why Does A Lithium Ion Battery Explode Unexpectedly?Lithium ion batteries are the most used type of batteries across several industries but, do you know what makes makes them hazardous? If you’re a researcher working with Li-ion batteries, you would know that one of the major reasons why most of the lithium-ion batteries explode is because of thermal runaway. What Is Thermal Runaway And Why It Is The Leading Cause Of Battery Explosions? Thermal runaway happens usually during charging. The temperature quickly rises to the melting point of the metallic lithium and causes a violent reaction. Another major reason behind thermal runaway is other microscopic metal particles coming in touch with different parts of the battery (this happens all the time in the battery assembly process), resulting in a short-circuit. Usually, a mild short circuit can cause an elevated self-discharge and little heat is generated because the discharging energy is very low. But, when enough microscopic metal particles converge on one spot, a major electrical short can develop and a sizable current will flow between the positive and negative plates. This causes the temperature to rise, leading to a thermal runaway, also referred to ‘venting with flame.’ During a thermal runaway, the high heat of the failing cell can propagate to the next cell, causing it to become thermally unstable as well. In some cases, a chain reaction occurs in which each cell disintegrates at its own timetable. Why Is Explosion Of Li-Ion Batteries A Major Issue For All?The smartphone in your pocket is powered by a Li-Ion battery. They are one of the most popular types of rechargeable batteries for portable electronics because of their high energy density, tiny memory effect, and low self-discharge. Beyond consumer electronics, Lithium Ion batteries are popular for military, electric vehicle and aerospace applications. For example, lithium ion batteries have replaced the conventional lead–acid batteries that have been used historically for golf carts and utility vehicles. The Global Lithium-Ion Battery Market size is expected reach $46.21 billion by 2022, with a CAGR of 10.8% during the period 2016-2022. For something which has become an integral part of our everyday life at such a fast pace, we would indeed be risking our lives having these batteries around us. Given their applications, they are not easily replaceable but if the thermal runaway problem could be solved, the balance would restore in paradise. How Can We Prevent Thermal Runaway In Lithium Ion Batteries?1. Introducing A Flame Retardant A flame retardant is a compound that inhibits, suppresses or delays the production of flames or prevents the spread of fire. Here they have the microencapsulated the flame retardant (usually a bromine compound) in high-density polyethylene and added water and a glycol compound to prepare the thermal fluid used. The glycol compound is used here as “antifreeze” (common glycol compounds used are ethylene glycol, diethylene glycol, and propylene glycol). Also, the invention is mostly discussed in light of EV batteries. A battery when called upon to power an electric vehicle heats up. Thermal fluid flows through the container and over the modules of the battery. In the event of an overcharge, or a car accident resulting in a battery puncture, the flame retardant in the thermal fluid acts to reduce the fire hazard. More precisely, bromine compound microcapsules rupture when rupture temperature is reached because of excess heat of the fire. The flame retardant is released from the microcapsules and acts to bring the fire under control. 2. Using Damage Initiating Devices In 2006, they filed a patent related to high elastic modulus polymer electrolytes suitable for preventing thermal runaway (US8703310). A different set of inventors has filed this patent (i.e. US’535) in 2013, about mitigating thermal runaway using damage-initiating materials or devices. More precisely, they have developed a thermal runaway shutdown mechanism that can be triggered either mechanically or thermally (or both), as battery damage happens (i.e., before or shortly after thermal runaway starts) and take care of the problem before it can even begin. Such predictive or instantaneous countermeasures are especially needed when a battery is subjected to impact or high-pressure (like an accident as I mentioned for the previous patent US’886 as well) and its internal structure damaged, causing internal shorting. The basic principle on which it operates is – as a mechanical load is applied to the battery, damage initiators can trigger widespread damage or destruction of the electrode so that the internal resistance increases significantly to mitigate thermal runaway even before it can happen. Here they have talked about two types of damage initiators – Passive damage initiators These initiators initiate cracking or voiding in electrodes upon impact, and such cracks and/or voids increase the internal impedance of the electrode and, thus, reduce heat generation associated with possible internal shorting. Such additives are known as cracks or voids initiators (CVIs). The electrode damages can be caused by debonding or stiffness mismatch of CVI-electrode interfaces, fracture, and rupture of CVI, etc. Examples of passive additives include solid or porous particles, solid or hollow/porous fibers, and tubes, etc. and they can be formed from carbon materials such as graphite, carbon nanotubes, activated carbons, carbon blacks, etc. Active damage initiator These initiators can produce a significant volume or shape change upon a mechanical or thermal loading. Active damage initiators can include solid or porous particles, solid or hollow beads, solid or hollow/porous fibers and tubes, etc. Active damage initiators can be formed from shape-memory alloys such as Ni—Ti, Ni—Ti—Pd, Ni—Ti—Pt, etc.
The chemicals that are released during thermal runaway can be toxic and in extreme cases, thermal runaway can cause electrical fires and/or batteries to explode. The ambient air temperature in the battery environment must also be properly maintained. Controlling these factors reduces the potential for thermal runaway. source:https://www.greyb.com/prevent-thermal-runaway-problem-li-ion-batteries/ |
A Guide to Choosing the Best 48V Lithium Golf Cart Battery
Would it be worth investing in a 48V ...
10 Exciting Ways To Use Your 12V Lithium Batteries
Back in 2016 when BSLBATT first began designing what would become the first drop-in replacemen...
BSLBATT Battery Company Receives Bulk Orders from North American Customers
BSLBATT®, a China Forklift battery manufacturer specializing in the material handling indust...
Fun Find Friday: BSLBATT Battery is coming to another great LogiMAT 2022
MEET US! VETTER’S EXHIBITION YEAR 2022! LogiMAT in Stuttgart: SMART – SUSTAINABLE – SAF...
Looking for new Distributors and Dealers for BSL Lithium Batteries
BSLBATT battery is a fast-paced, high-growth (200% YoY ) hi-tech company that is leading the a...
BSLBATT to Participate at MODEX 2022 on March 28-31 in Atlanta, GA
BSLBATT is one of the largest developers, manufacturers, and integrators of lithium-ion batter...
What makes the BSLBATT the Superior Lithium Battery for your Motive Power needs?
Electric forklift and Floor Cleaning Machines owners who seek the ultimate performance will fi...